CUTANA™ pAG-MNase for ChIC/CUT&RUN Workflows
{"url":"https://www.epicypher.com/products/epigenetics-reagents-and-assays/cutana-pag-mnase-for-chic-cut-and-run-workflows","add_this":[{"service":"facebook","annotation":""},{"service":"email","annotation":""},{"service":"print","annotation":""},{"service":"twitter","annotation":""},{"service":"linkedin","annotation":""}],"gtin":"","options":[{"id":1209,"type":"Configurable_PickList_Set","display_name":"Select Pack Size:","required":true,"condition":true,"state":"variant_option","values":[{"label":"50 Reactions","id":159,"data":"50 Reactions","selected":true},{"label":"250 Reactions","id":160,"data":"250 Reactions","selected":false}],"partial":"set-radio"}],"id":"694","bulk_discount_rates":[],"can_purchase":true,"meta_description":"CUTANA™ pAG-MNase for ChIC/CUT&RUN Workflows is a fusion of Proteins A and G to Micrococcal Nuclease. CUTANA pAG-MNase is recombinantly produced in E. coli.","category":["Epigenetics Kits and Reagents","Epigenetics Kits and Reagents/CUTANA™ ChIC / CUT&RUN Assays"],"AddThisServiceButtonMeta":"","main_image":{"data":"https://cdn11.bigcommerce.com/s-y9o92/images/stencil/{:size}/products/694/689/Screen_Shot_2020-02-12_at_11.01.55_AM__17144.1581530752.png?c=2","alt":"CUTANA™ pAG-MNase for ChIC/CUT&RUN Workflows"},"add_to_wishlist_url":"/wishlist.php?action=add&product_id=694","shipping":{"calculated":true},"num_reviews":0,"weight":"0.01 LBS","custom_fields":[{"id":"1174","name":"Internal Comment","value":"Excess in bottom of Venom"},{"id":"1175","name":"Internal Comment","value":"Bulk in Psylocke"}],"sku":"15-1016","description":"<div class=\"product-general-info\">\n <ul class=\"product-general-info__list-left\">\n <li class=\"product-general-info__list-item\">\n <strong>Type: </strong>Nuclease\n </li>\n <li class=\"product-general-info__list-item\">\n <strong>Mol Wgt: </strong>43.7 kDa\n </li>\n </ul>\n <ul class=\"product-general-info__list-right\">\n <li class=\"product-general-info__list-item\">\n <strong>Host: </strong><em>E. coli</em>\n </li>\n <li class=\"product-general-info__list-item\">\n <strong>Epitope Tag: </strong>6xHis\n </li>\n </ul>\n <ul class=\"product-general-info__list-right\">\n <li class=\"product-general-info__list-item\">\n <a href=\"#bioz\">\n <div\n id=\"w-s-3835-15-1016\"\n style=\"\n width: max-content;\n height: 58px;\n position: relative;\n overflow-y: hidden;\n \"></div>\n <div id=\"bioz-w-pb-15-1016-div\">\n <a\n id=\"bioz-w-pb-15-1016\"\n style=\"font-size: 12px; color: transparent\"\n href=\"https://www.bioz.com/\"\n target=\"_blank\">\n <img\n src=\"https://cdn.bioz.com/assets/favicon.png\"\n style=\"\n width: 11px;\n height: 11px;\n vertical-align: baseline;\n padding-bottom: 0px;\n margin-left: 0px;\n margin-bottom: 0px;\n float: none;\n display: none;\n \" />\n </a></div\n ></a>\n </li>\n </ul>\n</div>\n<div class=\"service_accordion product-droppdown\">\n <div class=\"container\">\n <div id=\"prodAccordion\">\n <div id=\"ProductDescription\" class=\"Block Panel current\">\n <h3 class=\"sub-title1\">Description</h3>\n <div\n class=\"ProductDescriptionContainer product-droppdown__section-description-specific\">\n <p>\n Available in 50 and 250 Reaction pack sizes. CUTANA™ pAG-MNase is\n the key reagent for performing Chromatin Immunocleavage (ChIC) [<a\n href=\"https://pubmed.ncbi.nlm.nih.gov/15469830/\"\n target=\"_blank\"\n rel=\"noopener noreferrer\"\n >Schmid et al., 2004</a\n >] and Cleavage Under Targets and Release Using Nuclease (CUT&RUN)\n [<a\n href=\"https://pubmed.ncbi.nlm.nih.gov/28079019/\"\n target=\"_blank\"\n rel=\"noopener noreferrer\"\n >Skene & Henikoff, 2017</a\n >,\n <a\n href=\"https://pubmed.ncbi.nlm.nih.gov/29651053/\"\n target=\"_blank\"\n rel=\"noopener noreferrer\"\n >Skene et al., 2018</a\n >].\n </p>\n\n <p\n style=\"\n background-color: #4698cb;\n color: #fff;\n padding: 1.3rem;\n text-align: center;\n border-radius: 12px;\n \">\n <a\n target=\"_blank\"\n style=\"color: #fff\"\n href=\"/products/epigenetics-reagents-and-assays/cutana-cut-and-run-library-prep-kit\"\n >Add our Library Prep Kit for a streamlined CUT&RUN workflow</a\n >\n </p>\n <p>\n As a fusion of Proteins A and G to Micrococcal Nuclease, CUTANA\n pAG-MNase is compatible with target antibodies from a broad spectrum\n of host species and is highly purified to remove contaminating\n <em>E. coli</em> DNA, which can complicate analysis at low cell\n numbers. This enzyme enables efficient mapping of chromatin features\n in ChIC/CUT&RUN, allowing for significant improvements in signal to\n noise at reduced cell inputs and sequencing depth compared to\n ChIP-seq.\n </p>\n </div>\n </div>\n </div>\n <div id=\"prodAccordion\">\n <div id=\"ProductDescription\" class=\"Block Panel current\">\n <h3 class=\"sub-title1\">Validation Data</h3>\n <div\n class=\"ProductDescriptionContainer product-droppdown__section-description-specific\">\n \n <section class=\"image-picker\">\n <div class=\"image-picker__left\">\n <div class=\"image-picker__main-content_active image-picker__main-content\">\n <div class=\"image-picker__header-content\">\n <button class=\"image-picker__left-arrow\">\n <svg\n class=\"image-picker__svg-left\"\n width=\"24\"\n height=\"24\"\n viewBox=\"0 0 24 24\">\n <path\n d=\"M16.67 0l2.83 2.829-9.339 9.175 9.339 9.167-2.83 2.829-12.17-11.996z\" />\n </svg>\n </button>\n <a\n href=\"/content/images/products/proteins/15-1016-gene-browser-tracks.jpeg\"\n target=\"blank\"\n class=\"image-picker__main-image-link\"\n ><img\n loading=\"lazy\"\n alt=\"15-1016-gene-browser-tracks\"\n src=\"/content/images/products/proteins/15-1016-gene-browser-tracks.jpeg\"\n class=\"image-picker__main-image\" />\n <span class=\"image-picker__main-image-caption\"\n >(Click to enlarge)</span\n ></a\n >\n <button class=\"image-picker__right-arrow\">\n <svg\n class=\"image-picker__svg-right\"\n width=\"24\"\n height=\"24\"\n viewBox=\"0 0 24 24\">\n <path\n d=\"M7.33 24l-2.83-2.829 9.339-9.175-9.339-9.167 2.83-2.829 12.17 11.996z\" />\n </svg>\n </button>\n </div>\n <p>\n <span class=\"image-picker__span-content\"\n ><strong>Figure 1: CUT&RUN gene browser tracks </strong\n ><br />\n CUT&RUN was performed as described above. Data verifies low\n non-specific MNase digestion with the absence of peaks in\n the IgG track, an expected H3K4me3 profile with sharp\n promoter peaks, and broad peaks in heterochromatin regions\n consistent with H3K27me3. Image was generated using the\n Integrative Genomics Viewer (IGV, Broad Institute).\n </span>\n </p>\n </div>\n <div class=\"image-picker__main-content\">\n <div class=\"image-picker__header-content\">\n <button class=\"image-picker__left-arrow\">\n <svg\n class=\"image-picker__svg-left\"\n width=\"24\"\n height=\"24\"\n viewBox=\"0 0 24 24\">\n <path\n d=\"M16.67 0l2.83 2.829-9.339 9.175 9.339 9.167-2.83 2.829-12.17-11.996z\" />\n </svg>\n </button>\n <a\n href=\"/content/images/products/proteins/15-1016-size-distribution.jpeg\"\n target=\"blank\"\n class=\"image-picker__main-image-link\"\n ><img\n loading=\"lazy\"\n alt=\"15-1016\"\n src=\"/content/images/products/proteins/15-1016-size-distribution.jpeg\"\n class=\"image-picker__main-image\" />\n <span class=\"image-picker__main-image-caption\"\n >(Click to enlarge)</span\n ></a\n >\n <button class=\"image-picker__right-arrow\">\n <svg\n class=\"image-picker__svg-right\"\n width=\"24\"\n height=\"24\"\n viewBox=\"0 0 24 24\">\n <path\n d=\"M7.33 24l-2.83-2.829 9.339-9.175-9.339-9.167 2.83-2.829 12.17 11.996z\" />\n </svg>\n </button>\n </div>\n <p>\n <span class=\"image-picker__span-content\"\n ><strong\n >Figure 2: Size distribution of released chromatin</strong\n ><br />\n CUT&RUN was performed as described above. Excised DNA is\n highly enriched for mononucleosomes (peaks at ~300 bp\n represent 150 bp nucleosomes + sequencing adapters).\n </span>\n </p>\n </div>\n <div class=\"image-picker__main-content\">\n <div class=\"image-picker__header-content\">\n <button class=\"image-picker__left-arrow\">\n <svg\n class=\"image-picker__svg-left\"\n width=\"24\"\n height=\"24\"\n viewBox=\"0 0 24 24\">\n <path\n d=\"M16.67 0l2.83 2.829-9.339 9.175 9.339 9.167-2.83 2.829-12.17-11.996z\" />\n </svg>\n </button>\n <a\n href=\"/content/images/products/proteins/15-1016-heatmaps.jpeg\"\n target=\"blank\"\n class=\"image-picker__main-image-link\">\n <img\n loading=\"lazy\"\n alt=\"15-1016-heatmaps\"\n src=\"/content/images/products/proteins/15-1016-heatmaps.jpeg\"\n class=\"image-picker__main-image\" />\n <span class=\"image-picker__main-image-caption\"\n >(Click to enlarge)</span\n >\n </a>\n <button class=\"image-picker__right-arrow\">\n <svg\n class=\"image-picker__svg-right\"\n width=\"24\"\n height=\"24\"\n viewBox=\"0 0 24 24\">\n <path\n d=\"M7.33 24l-2.83-2.829 9.339-9.175-9.339-9.167 2.83-2.829 12.17 11.996z\" />\n </svg>\n </button>\n </div>\n <p>\n <span class=\"image-picker__span-content\">\n <strong>Figure 3: CUT&RUN genome-wide heatmaps</strong\n ><br />\n CUT&RUN was performed as described above. Heatmaps show\n CUT&RUN signal aligned to annotated transcription start\n sites (TSS, +/- 2kb). High and low signal are ranked by\n intensity (top to bottom) and colored such that red\n indicates high localized enrichment and blue denotes\n background signal. Gene rows in each heatmap are aligned and\n sorted from high to low signal relative to H3K4me3 (middle).\n </span>\n </p>\n </div>\n <div class=\"image-picker__main-content\">\n <div class=\"image-picker__header-content\">\n <button class=\"image-picker__left-arrow\">\n <svg\n class=\"image-picker__svg-left\"\n width=\"24\"\n height=\"24\"\n viewBox=\"0 0 24 24\">\n <path\n d=\"M16.67 0l2.83 2.829-9.339 9.175 9.339 9.167-2.83 2.829-12.17-11.996z\" />\n </svg>\n </button>\n <a\n href=\"/content/images/products/proteins/15-1016-protein-gel-data.jpeg\"\n target=\"blank\"\n class=\"image-picker__main-image-link\">\n <img\n loading=\"lazy\"\n alt=\"15-1016-protein-gel-data\"\n src=\"/content/images/products/proteins/15-1016-protein-gel-data.jpeg\"\n class=\"image-picker__main-image\" />\n <span class=\"image-picker__main-image-caption\"\n >(Click to enlarge)</span\n >\n </a>\n <button class=\"image-picker__right-arrow\">\n <svg\n class=\"image-picker__svg-right\"\n width=\"24\"\n height=\"24\"\n viewBox=\"0 0 24 24\">\n <path\n d=\"M7.33 24l-2.83-2.829 9.339-9.175-9.339-9.167 2.83-2.829 12.17 11.996z\" />\n </svg>\n </button>\n </div>\n <p>\n <span class=\"image-picker__span-content\">\n <strong>Figure 4: Protein gel data</strong><br />\n CUTANA™ pAG-MNase (1 µg) was resolved via SDS-PAGE and\n stained with Coomassie blue. The migration and molecular\n weight of the protein standards are indicated.\n </span>\n </p>\n </div>\n <div class=\"image-picker__main-content\">\n <div class=\"image-picker__header-content\">\n <button class=\"image-picker__left-arrow\">\n <svg\n class=\"image-picker__svg-left\"\n width=\"24\"\n height=\"24\"\n viewBox=\"0 0 24 24\">\n <path\n d=\"M16.67 0l2.83 2.829-9.339 9.175 9.339 9.167-2.83 2.829-12.17-11.996z\" />\n </svg>\n </button>\n <a\n href=\"/content/images/products/methods/15-1016-cut-and-run-methods.png\"\n target=\"blank\"\n class=\"image-picker__main-image-link\">\n <img\n loading=\"lazy\"\n alt=\"15-1016-protein-gel-data\"\n src=\"/content/images/products/methods/15-1016-cut-and-run-methods.png\"\n class=\"image-picker__main-image\" />\n <span class=\"image-picker__main-image-caption\"\n >(Click to enlarge)</span\n >\n </a>\n <button class=\"image-picker__right-arrow\">\n <svg\n class=\"image-picker__svg-right\"\n width=\"24\"\n height=\"24\"\n viewBox=\"0 0 24 24\">\n <path\n d=\"M7.33 24l-2.83-2.829 9.339-9.175-9.339-9.167 2.83-2.829 12.17 11.996z\" />\n </svg>\n </button>\n </div>\n <p>\n <span class=\"image-picker__span-content\">\n <strong>CUT&RUN methods</strong><br/>\n CUT&RUN was performed on 500k K562 cells with 0.5 µg of either IgG (EpiCypher <a href=\"/products/nucleosomes/snap-cutana-spike-in-controls/cutana-rabbit-igg-cut-run-negative-control-antibody\"> 13-0042</a>), \n H3K4me3 (EpiCypher <a href=\"/products/antibodies/snap-chip-certified-antibodies/histone-h3k4me3-antibody-snap-chip-certified-cutana-cut-run-compatible\"> 13-0041</a>), or H3K27me3 (<a href=\"https://www.thermofisher.com/antibody/product/H3K27me3-Antibody-clone-G-299-10-Monoclonal/MA5-11198\">ThermoFisher MA5-11198</a>) antibodies \n using CUTANA™ pAG-MNase (1:20 dilution) and the CUTANA™ ChIC/CUT&RUN Kit v3 (EpiCypher <a href=\"/products/epigenetics-reagents-and-assays/cutana-chic-cut-and-run-kit\"> 14-1048</a>). \n Library preparation was performed with 5 ng of DNA (or the total amount recovered if less than 5 ng) \n using the CUTANA™ CUT&RUN Library Prep Kit (EpiCypher <a href=\"/products/epigenetics-reagents-and-assays/cutana-cut-and-run-library-prep-kit\"> 14-1001/14-1002</a>). Both kit protocols were adapted for\n high throughput Tecan liquid handling. Libraries were run on an Illumina NextSeq2000 with paired-end sequencing (2x50 bp). \n Sample sequencing depth was 3.6 million reads (IgG), 4.3 million reads (H3K4me3), and 5.2 million reads (H3K27me3).\n Data were aligned to the hg19 genome using Bowtie2. Data were filtered to remove duplicates, multi-aligned reads, \n and ENCODE DAC Exclusion List regions.\n </span>\n </p>\n </div>\n\n </div>\n <aside class=\"image-picker__right\">\n <div class=\"image-picker__gallery\">\n <img\n loading=\"lazy\"\n alt=\"15-1016-gene-browser-tracks\"\n src=\"/content/images/products/proteins/15-1016-gene-browser-tracks.jpeg\"\n width=\"200\"\n class=\"image-picker__side-image \"\n role=\"button\" />\n <img\n loading=\"lazy\"\n alt=\"15-1016-size-distribution\"\n src=\"/content/images/products/proteins/15-1016-size-distribution.jpeg\"\n class=\"image-picker__side-image\"\n role=\"button\" />\n <img\n loading=\"lazy\"\n alt=\"15-1016-heatmaps\"\n src=\"/content/images/products/proteins/15-1016-heatmaps.jpeg\"\n class=\"image-picker__side-image\"\n role=\"button\" />\n <img\n loading=\"lazy\"\n alt=\"15-1016-protein-gel-data\"\n src=\"/content/images/products/proteins/15-1016-protein-gel-data.jpeg\"\n class=\"image-picker__side-image\"\n role=\"button\" />\n <img\n loading=\"lazy\"\n alt=\"15-1016-cut-and-run-methods\"\n src=\"/content/images/products/methods/15-1016-cut-and-run-methods.png\"\n class=\"image-picker__side-image image-picker__side-image_active\"\n role=\"button\" />\n </div>\n </aside>\n </section>\n </div>\n </div>\n </div>\n <div id=\"prodAccordion\">\n <div id=\"ProductDescription\" class=\"Block Panel\">\n <h3 class=\"sub-title1\">Recommended Accessory Products</h3>\n <div\n class=\"ProductDescriptionContainer product-droppdown__section-description\">\n <table class=\"epicypher-table\">\n <tr>\n <th>Item</th>\n <th>Cat. No.</th>\n </tr>\n\n <tr>\n <td>CUTANA™ CUT&RUN Library Prep Kit</td>\n <td>\n <a\n href=\"/products/epigenetics-reagents-and-assays/cutana-cut-and-run-library-prep-kit\"\n >14-1001</a\n >\n </td>\n </tr>\n <tr>\n <td>CUTANA™ DNA Purification Kit</td>\n <td>\n <a\n href=\"/products/epigenetics-reagents-and-assays/cutana-dna-purification-kit\"\n >14-0050</a\n >\n </td>\n </tr>\n\n <tr>\n <td>CUTANA™ Concanavalin A Conjugated Paramagnetic Beads</td>\n <td>\n <a\n href=\"/products/epigenetics-kits-and-reagents/cutana-chic-cut-run-assays/cutana-concanavalin-a-conjugated-paramagnetic-beads\"\n >21-1401</a\n >\n </td>\n </tr>\n\n <tr>\n <td>SNAP-CUTANA™ K-MetStat Panel</td>\n <td>\n <a href=\"/products/nucleosomes/snap-cutana-k-metstat-panel\"\n >19-1002</a\n >\n </td>\n </tr>\n\n <tr>\n <td>CUT&RUN Antibodies</td>\n <td>\n <a href=\"/products/antibodies/cutana-cut-and-run-antibodies\"\n >See the list</a\n >\n </td>\n </tr>\n\n <tr>\n <td>CUTANA™ <em>E. coli</em> Spike-in DNA</td>\n <td>\n <a\n href=\"/products/nucleosomes/snap-cutana-spike-in-controls/cutana-e-coli-spike-in-dna\"\n >18-1401</a\n >\n </td>\n </tr>\n\n <tr>\n <td>Magnetic Separation Rack, 0.2 mL Tubes</td>\n <td>\n <a\n href=\"/products/epigenetics-reagents-and-assays/cutana-chic-cut-run-assays/magnetic-separation-rack-0-2-ml-tubes\"\n >10-0008</a\n >\n </td>\n </tr>\n\n <tr>\n <td>Magnetic Separation Rack, 1.5 mL Tubes</td>\n <td>\n <a\n href=\"/products/epigenetics-reagents-and-assays/magnetic-separation-rack-1-5-ml-tubes\"\n >10-0012</a\n >\n </td>\n </tr>\n\n <tr>\n <td>CUTANA™ CUT&RUN 8-strip 0.2 mL Tubes</td>\n <td>\n <a\n href=\"/products/epigenetics-reagents-and-assays/cutana-chic-cut-run-assays/cutana-cut-run-8-strip-0-2-ml-tubes\"\n >10-0009</a\n >\n </td>\n </tr>\n<tr>\n <td id=\"specific-row\">\n CUTANA™ Nuclei Extraction Buffer \n </td>\n <td>\n <a\n href=\"https://www.epicypher.com/products.php?product=CUTANA%E2%84%A2-Nuclei-Extraction-Buffer&showHidden=true\"\n >21-1026</a\n >\n </td>\n </tr>\n </table>\n </div>\n </div>\n </div>\n <div id=\"prodAccordion\">\n <div id=\"ProductDescription\" class=\"Block Panel\">\n <h3 class=\"sub-title1\">Technical Information</h3>\n <div\n class=\"ProductDescriptionContainer product-droppdown__section-description\">\n <div class=\"product-tech-info\">\n <div class=\"product-tech-info__line-item\">\n <div class=\"product-tech-info__line-item-left\">\n <b>Storage</b>\n </div>\n <div class=\"product-tech-info__line-item-right\">\n Stable for one year at -20°C from date of receipt. The protein\n is not subject to freeze/thaw under these conditions.\n </div>\n </div>\n <div class=\"product-tech-info__line-item\">\n <div class=\"product-tech-info__line-item-left\">\n <b>Formulation</b>\n </div>\n <div class=\"product-tech-info__line-item-right\">\n 10 mM Tris HCl pH 7.5, 150 mM NaCl, 1 mM EDTA, and 50% glycerol.\n </div>\n </div>\n </div>\n </div>\n </div>\n </div>\n <div id=\"prodAccordion\">\n <div id=\"ProductDescription\" class=\"Block Panel\">\n <h3 class=\"sub-title1\">Application Notes</h3>\n <div\n class=\"ProductDescriptionContainer product-droppdown__section-description\">\n <p>\n Add 2.5 µL of the supplied enzyme to a 50 µL CUT&RUN reaction (20X\n dilution). For detailed applications and uses of this product,\n please see our\n <a href=\"/content/documents/protocols/cutana-cut&run-protocol.pdf\"\n >CUT&RUN protocol</a\n >.\n </p>\n </div>\n </div>\n </div>\n <div id=\"prodAccordion\">\n <div id=\"ProductDescription\" class=\"Block Panel\">\n <h3 class=\"sub-title1\">Documents & Resources</h3>\n <div\n class=\"ProductDescriptionContainer product-droppdown__section-description\">\n <div class=\"product-documents\">\n <a\n href=\"/content/documents/tds/15-1016.pdf\"\n target=\"blank\"\n class=\"product-documents__link\">\n <svg\n version=\"1.1\"\n id=\"Layer_1\"\n xmlns=\"http://www.w3.org/2000/svg\"\n xmlns:xlink=\"http://www.w3.org/1999/xlink\"\n x=\"0px\"\n y=\"0px\"\n viewBox=\"0 0 228 240\"\n style=\"enable-background: new 0 0 228 240\"\n xml:space=\"preserve\"\n class=\"product-documents__icon\"\n alt=\"15-1016 Datasheet\">\n <g>\n <path\n class=\"product-documents__svg-pdf\"\n d=\"M191.92,68.77l-47.69-47.69c-1.33-1.33-3.12-2.08-5.01-2.08H45.09C41.17,19,38,22.17,38,26.09v184.36\n c0,3.92,3.17,7.09,7.09,7.09h141.82c3.92,0,7.09-3.17,7.09-7.09V73.8C194,71.92,193.25,70.1,191.92,68.77z M177.65,77.06h-41.7\n v-41.7L177.65,77.06z M178.05,201.59H53.95V34.95h66.92v47.86c0,5.14,4.17,9.31,9.31,9.31h47.86V201.59z\" />\n </g>\n <rect\n x=\"20\"\n y=\"112\"\n class=\"product-documents__svg-background\"\n width=\"146\"\n height=\"76\" />\n <g>\n <path\n class=\"product-documents__svg-pdf\"\n d=\"M23.83,125.68h22.36c5.29,0,9.41,1.33,12.35,4c2.94,2.67,4.42,6.39,4.42,11.18c0,4.78-1.47,8.51-4.42,11.18\n c-2.94,2.67-7.06,4-12.35,4H34.59v18.29H23.83V125.68z M44.81,147.9c5.38,0,8.07-2.32,8.07-6.97c0-2.39-0.67-4.16-2-5.31\n c-1.33-1.15-3.36-1.73-6.07-1.73H34.59v14.01H44.81z\" />\n <path\n class=\"product-documents__svg-pdf\"\n d=\"M69.92,125.68h18.91c5.29,0,9.84,0.97,13.66,2.9c3.82,1.93,6.74,4.72,8.76,8.35\n c2.02,3.63,3.04,7.98,3.04,13.04c0,5.06-1,9.42-3,13.08c-2,3.66-4.91,6.45-8.73,8.38c-3.82,1.93-8.4,2.9-13.73,2.9H69.92V125.68z\n M88.07,165.63c10.35,0,15.52-5.22,15.52-15.66c0-10.4-5.17-15.59-15.52-15.59h-7.38v31.26H88.07z\" />\n <path\n class=\"product-documents__svg-pdf\"\n d=\"M122.57,125.68h32.84v8.49h-22.22v11.18h20.84v8.49h-20.84v20.49h-10.63V125.68z\" />\n </g>\n </svg>\n <span class=\"product-documents__info\">Technical Datasheet</span>\n </a>\n </div>\n <div class=\"product-documents\">\n <a\n href=\"/content/documents/protocols/cutana-cut&run-protocol.pdf\"\n target=\"blank\"\n class=\"product-documents__link\">\n <svg\n version=\"1.1\"\n id=\"Layer_1\"\n xmlns=\"http://www.w3.org/2000/svg\"\n xmlns:xlink=\"http://www.w3.org/1999/xlink\"\n x=\"0px\"\n y=\"0px\"\n viewBox=\"0 0 228 240\"\n style=\"enable-background: new 0 0 228 240\"\n xml:space=\"preserve\"\n class=\"product-documents__icon\"\n alt=\"CUTANA CUT&RUN Protocol\">\n <g>\n <path\n class=\"product-documents__svg-pdf\"\n d=\"M191.92,68.77l-47.69-47.69c-1.33-1.33-3.12-2.08-5.01-2.08H45.09C41.17,19,38,22.17,38,26.09v184.36\n c0,3.92,3.17,7.09,7.09,7.09h141.82c3.92,0,7.09-3.17,7.09-7.09V73.8C194,71.92,193.25,70.1,191.92,68.77z M177.65,77.06h-41.7\n v-41.7L177.65,77.06z M178.05,201.59H53.95V34.95h66.92v47.86c0,5.14,4.17,9.31,9.31,9.31h47.86V201.59z\" />\n </g>\n <rect\n x=\"20\"\n y=\"112\"\n class=\"product-documents__svg-background\"\n width=\"146\"\n height=\"76\" />\n <g>\n <path\n class=\"product-documents__svg-pdf\"\n d=\"M23.83,125.68h22.36c5.29,0,9.41,1.33,12.35,4c2.94,2.67,4.42,6.39,4.42,11.18c0,4.78-1.47,8.51-4.42,11.18\n c-2.94,2.67-7.06,4-12.35,4H34.59v18.29H23.83V125.68z M44.81,147.9c5.38,0,8.07-2.32,8.07-6.97c0-2.39-0.67-4.16-2-5.31\n c-1.33-1.15-3.36-1.73-6.07-1.73H34.59v14.01H44.81z\" />\n <path\n class=\"product-documents__svg-pdf\"\n d=\"M69.92,125.68h18.91c5.29,0,9.84,0.97,13.66,2.9c3.82,1.93,6.74,4.72,8.76,8.35\n c2.02,3.63,3.04,7.98,3.04,13.04c0,5.06-1,9.42-3,13.08c-2,3.66-4.91,6.45-8.73,8.38c-3.82,1.93-8.4,2.9-13.73,2.9H69.92V125.68z\n M88.07,165.63c10.35,0,15.52-5.22,15.52-15.66c0-10.4-5.17-15.59-15.52-15.59h-7.38v31.26H88.07z\" />\n <path\n class=\"product-documents__svg-pdf\"\n d=\"M122.57,125.68h32.84v8.49h-22.22v11.18h20.84v8.49h-20.84v20.49h-10.63V125.68z\" />\n </g>\n </svg>\n <span class=\"product-documents__info\"\n >CUT&RUN Protocol for Histone PTMs and Chomatin-Interacting\n Proteins</span\n >\n </a>\n </div>\n <div class=\"product-documents\">\n <a\n href=\"/resources/protocols/cutana-cut-and-run-protocol/\"\n target=\"blank\"\n class=\"product-documents__link\">\n <svg\n version=\"1.1\"\n id=\"Layer_1\"\n xmlns=\"http://www.w3.org/2000/svg\"\n xmlns:xlink=\"http://www.w3.org/1999/xlink\"\n x=\"0px\"\n y=\"0px\"\n viewBox=\"0 0 228 240\"\n style=\"enable-background: new 0 0 228 240\"\n xml:space=\"preserve\"\n class=\"product-documents__icon\"\n alt=\"CUTANAâ„¢ CUT&RUN Protocol\">\n <g>\n <path\n class=\"product-documents__svg-link\"\n d=\"M130.3,157.06c-0.77-0.76-2-0.76-2.77,0l-28.45,28.45c-13.17,13.17-35.41,14.57-49.95,0\n c-14.57-14.57-13.17-36.78,0-49.95l28.45-28.45c0.76-0.76,0.76-2.01,0-2.77l-9.75-9.75c-0.77-0.76-2-0.76-2.77,0l-28.45,28.45\n c-20.71,20.71-20.71,54.23,0,74.92s54.23,20.71,74.92,0L140,169.52c0.76-0.76,0.76-2.01,0-2.77L130.3,157.06z M193.62,41\n c-20.71-20.71-54.23-20.71-74.92,0L90.22,69.45c-0.76,0.77-0.76,2,0,2.77l9.72,9.72c0.76,0.76,2.01,0.76,2.77,0l28.45-28.45\n c13.17-13.17,35.41-14.57,49.95,0c14.57,14.57,13.17,36.78,0,49.95l-28.45,28.45c-0.76,0.77-0.76,2,0,2.77l9.75,9.75\n c0.76,0.76,2.01,0.76,2.77,0l28.45-28.45C214.31,95.23,214.31,61.71,193.62,41L193.62,41z M139.14,85.29c-0.77-0.76-2-0.76-2.77,0\n l-55.46,55.43c-0.76,0.77-0.76,2,0,2.77l9.7,9.7c0.76,0.76,2.01,0.76,2.77,0l55.43-55.43c0.76-0.76,0.76-2.01,0-2.77L139.14,85.29z\n \" />\n </g>\n </svg>\n <span class=\"product-documents__info\"\n >Previous CUT&RUN Protocol versions</span\n >\n </a>\n </div>\n </div>\n </div>\n </div>\n <div id=\"prodAccordion\">\n <div id=\"ProductDescription\" class=\"Block Panel current\">\n <h3 id=\"bioz\" class=\"sub-title1\">Product References</h3>\n <div\n class=\"ProductDescriptionContainer product-droppdown__section-description-specific\">\n <object\n id=\"wobj-3835-15-1016-q\"\n type=\"text/html\"\n data=\"https://www.bioz.com/v_widget_6_0/3835/15-1016/?ex=1\"\n style=\"width: 100%; height: 193px\"></object>\n <div id=\"bioz-w-pb-3835-15-1016-q-div\" style=\"width: 100%\">\n <a\n id=\"bioz-w-pb-3835-15-1016-q\"\n style=\"font-size: 12px; text-decoration: none; color: #4698cb\"\n href=\"https://www.bioz.com/\"\n target=\"_blank\"\n ><img\n src=\"https://cdn.bioz.com/assets/favicon.png\"\n style=\"\n width: 11px;\n height: 11px;\n vertical-align: baseline;\n padding-bottom: 0px;\n margin-left: 0px;\n margin-bottom: 0px;\n float: none;\n \" />\n Powered by Bioz</a\n >\n <a\n style=\"\n font-size: 12px;\n text-decoration: none;\n float: right;\n color: transparent;\n \"\n href=\"https://www.bioz.com/result/15-1016/product/EpiCypher/?cn=14-1048\"\n target=\"_blank\">\n See more details on Bioz</a\n >\n </div>\n </div>\n </div>\n </div>\n </div>\n</div>\n\n<script>\n $(document).ready(function () {\n var widget_micro_obj = new v_widget_obj('s', [1]);\n widget_micro_obj.request_catalog_number_widget_data_internal(\n '15-1016',\n '15-1016'\n );\n });\n</script>\n\n\n<script>\n $(document).ready(function () {\n const mainDiv = document.getElementsByClassName(\"bioz-w-container\");\n const kidDiv = document.getElementsByClassName(\"bioz-w-table-column\");\n mainDiv.style.overflowX = \"scroll\";\n kidDiv.style.overflow = \"auto\";\n document.getElementsByTagName(\"body\").getElementsByTagName(\"HTML\").style.overflowX = \"visible\";\n });\n</script>\n\n<style>\n html, body{\n overflow-x: visible !important;\n }\n .form-field-title .required-text {\n display: none !important;\n }\n\n .form-label {\n padding-left: 2rem;\n }\n\n .form-label-text {\n margin: 0;\n margin-left: 0 !important;\n }\n\n /* //////////// */\n\n /* BIOZ */\n td table {\n margin: 0;\n padding: 6px !important;\n }\n\n span.bioz-w-parent-hover {\n font-size: 15px;\n }\n\n td .bioz-w-tooltipx {\n padding-top: 0 !important;\n }\n /* .bioz-w-parent-hover:hover {\n text-decoration: underline !important;\n } */\n\n /* /////////// */\n\n /* .product-general-info {\n gap: 0rem;\n flex-wrap: wrap;\n} */\n\ndiv.bioz-w-table-column {\n overflow-x: auto !important;\n}\n\n.bioz-w-container {\n overflow-x: scroll !important;\n}\n@media only screen and (max-width: 1024px)\n{\n .product-general-info .product-general-info__list-left {\n width: 32% ;\n }\n}\n\n@media only screen and (max-width: 767px)\n{\n .product-general-info {\n -ms-flex-direction: column;\n flex-direction: column;\n gap: 0;\n }\n}\n\n\n .form-field-title .required-text {\n display: none !important;\n }\n .form-label {\n padding-left: 2rem;\n }\n .form-label-text {\n margin: 0;\n margin-left: 0 !important;\n }\n\n .bioz-w-header td {\n padding: 0;\n }\n\n .epicypher-table table {\n margin-right: auto;\n margin-left: auto;\n width: 100%;\n border-top: 1px solid lightgray;\n }\n\n .epicypher-table th {\n color: #3b3a48;\n }\n .epicypher-table td {\n color: #3b3a48;\n font-size: 0.9rem;\n }\n .epicypher-table td a {\n text-decoration: underline;\n }\n .epicypher-table td,\n th {\n border: 0.5px solid lightgray;\n text-align: left;\n padding: 8px;\n /* white-space: nowrap; */\n }\n .epicypher-table tr:nth-child(even) {\n background-color: #f9f9f9;\n }\n .epicypher-table td:first-child {\n border-left: 1px solid lightgray;\n }\n\n /* Tablet */\n\n @media only screen and (max-width: 1024px) {\n /* Force table to not be like tables anymore */\n .epicypher-table th {\n display: none !important;\n }\n\n /* BIOZ */\n td.bioz-w-tooltipx::before {\n display: none;\n }\n\n /* Hide table headers (but not display: none;, for accessibility) */\n .epicypher-table thead tr {\n position: absolute;\n top: -9999px;\n left: -9999px;\n }\n\n .epicypher-table tr {\n border: 1px solid #ccc;\n }\n\n .epicypher-table td {\n /* Behave like a \"row\" */\n /* border: none; */\n border-bottom: 1px solid #eee;\n position: relative;\n padding-left: 30%;\n white-space: inherit;\n }\n\n .epicypher-table td:before {\n /* Now like a table header */\n position: absolute;\n /* Top/left values mimic padding */\n top: 6px;\n left: 6px;\n width: 45%;\n padding-right: 10px;\n white-space: nowrap;\n font-weight: 700;\n }\n\n /*\n Label the data\n */\n .epicypher-table td:nth-of-type(1):before {\n content: 'Item';\n }\n td:nth-of-type(2):before {\n content: 'Cat. No.';\n }\n /* BIOZ */\n }\n\n @media screen and (min-width: 768px) {\n .products-featured .container,\n .products-featured .product-tabs,\n .products-related .container,\n .products-related .product-tabs {\n padding-left: 30px !important;\n padding-right: 50px;\n max-width: 1170px !important;\n }\n .bioz-w-header,\n .bioz-w-parent-hover td table tbody tr {\n display: inherit;\n }\n }\n\n @media screen and (max-width: 767px) {\n .custom-title-description {\n font-size: 19px !important;\n }\n .section-title {\n font-size: 19px !important;\n padding-left: 1rem;\n }\n }\n</style>\n","tags":[],"warranty":"","price":{"without_tax":{"formatted":"$335.00","value":335,"currency":"USD"},"tax_label":"Sales Tax"},"detail_messages":"","availability":"","page_title":"CUTANA™ pAG-MNase for ChIC/CUT&RUN Workflows | EpiCypher","cart_url":"https://www.epicypher.com/cart.php","max_purchase_quantity":0,"mpn":"","upc":null,"shipping_messages":[],"rating":0,"meta_keywords":"pag-mnase, CUT&RUN, CUT and RUN, genomic mapping, ChIP-Seq","show_quantity_input":1,"title":"CUTANA™ pAG-MNase for ChIC/CUT&RUN Workflows","gift_wrapping_available":false,"min_purchase_quantity":0,"customizations":[],"images":[{"data":"https://cdn11.bigcommerce.com/s-y9o92/images/stencil/{:size}/products/694/689/Screen_Shot_2020-02-12_at_11.01.55_AM__17144.1581530752.png?c=2","alt":"CUTANA™ pAG-MNase for ChIC/CUT&RUN Workflows"}]}
Description
Available in 50 and 250 Reaction pack sizes. CUTANA™ pAG-MNase is the key reagent for performing Chromatin Immunocleavage (ChIC) [Schmid et al., 2004] and Cleavage Under Targets and Release Using Nuclease (CUT&RUN) [Skene & Henikoff, 2017, Skene et al., 2018].
Add our Library Prep Kit for a streamlined CUT&RUN workflow
As a fusion of Proteins A and G to Micrococcal Nuclease, CUTANA pAG-MNase is compatible with target antibodies from a broad spectrum of host species and is highly purified to remove contaminating E. coli DNA, which can complicate analysis at low cell numbers. This enzyme enables efficient mapping of chromatin features in ChIC/CUT&RUN, allowing for significant improvements in signal to noise at reduced cell inputs and sequencing depth compared to ChIP-seq.
Validation Data
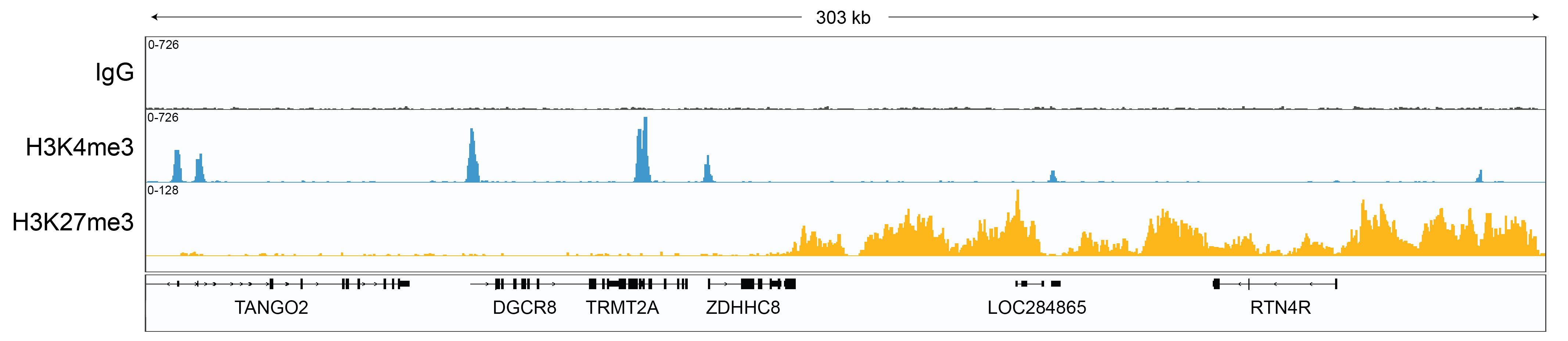
Figure 1: CUT&RUN gene browser tracks
CUT&RUN was performed as described above. Data verifies low
non-specific MNase digestion with the absence of peaks in
the IgG track, an expected H3K4me3 profile with sharp
promoter peaks, and broad peaks in heterochromatin regions
consistent with H3K27me3. Image was generated using the
Integrative Genomics Viewer (IGV, Broad Institute).
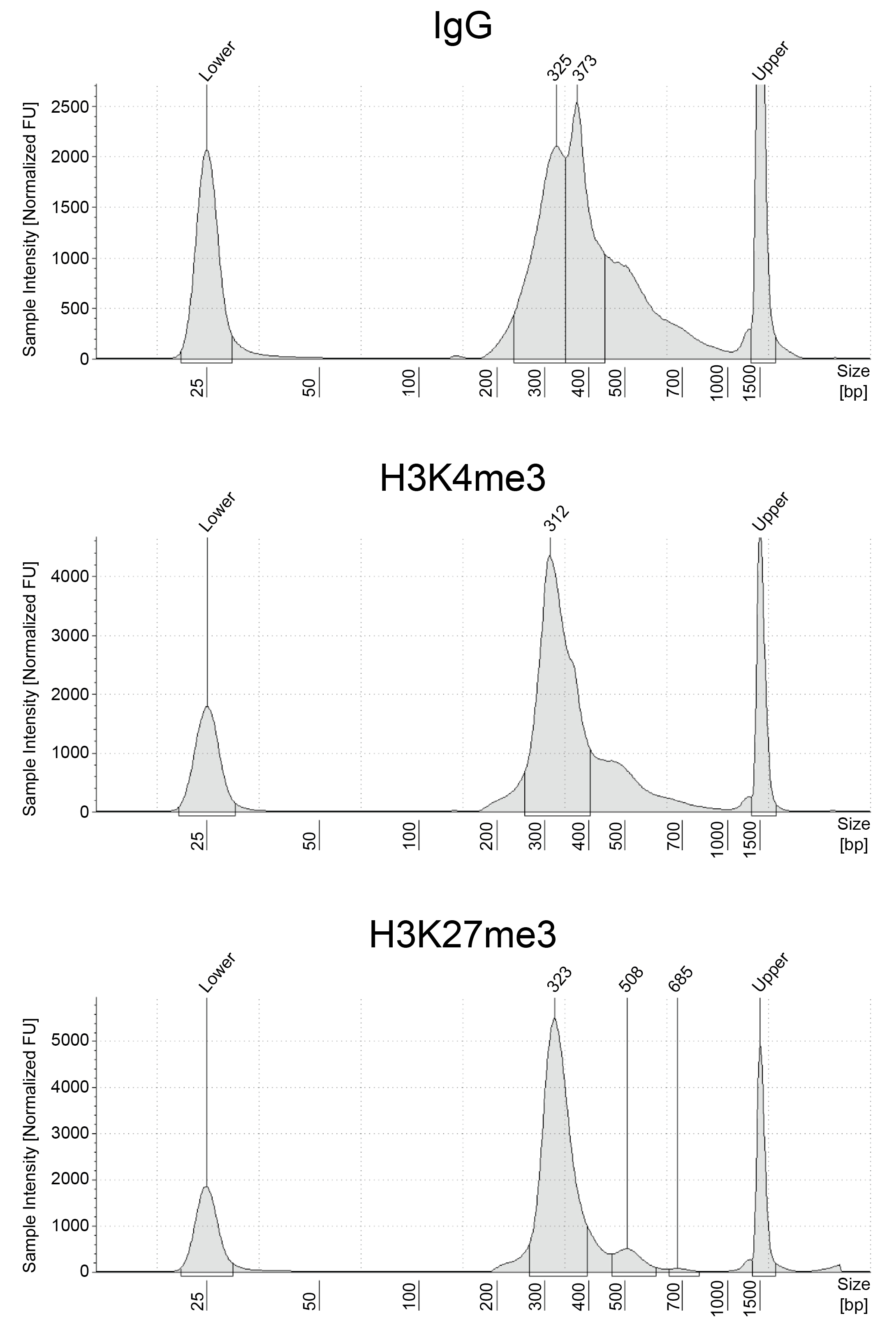
Figure 2: Size distribution of released chromatin
CUT&RUN was performed as described above. Excised DNA is
highly enriched for mononucleosomes (peaks at ~300 bp
represent 150 bp nucleosomes + sequencing adapters).
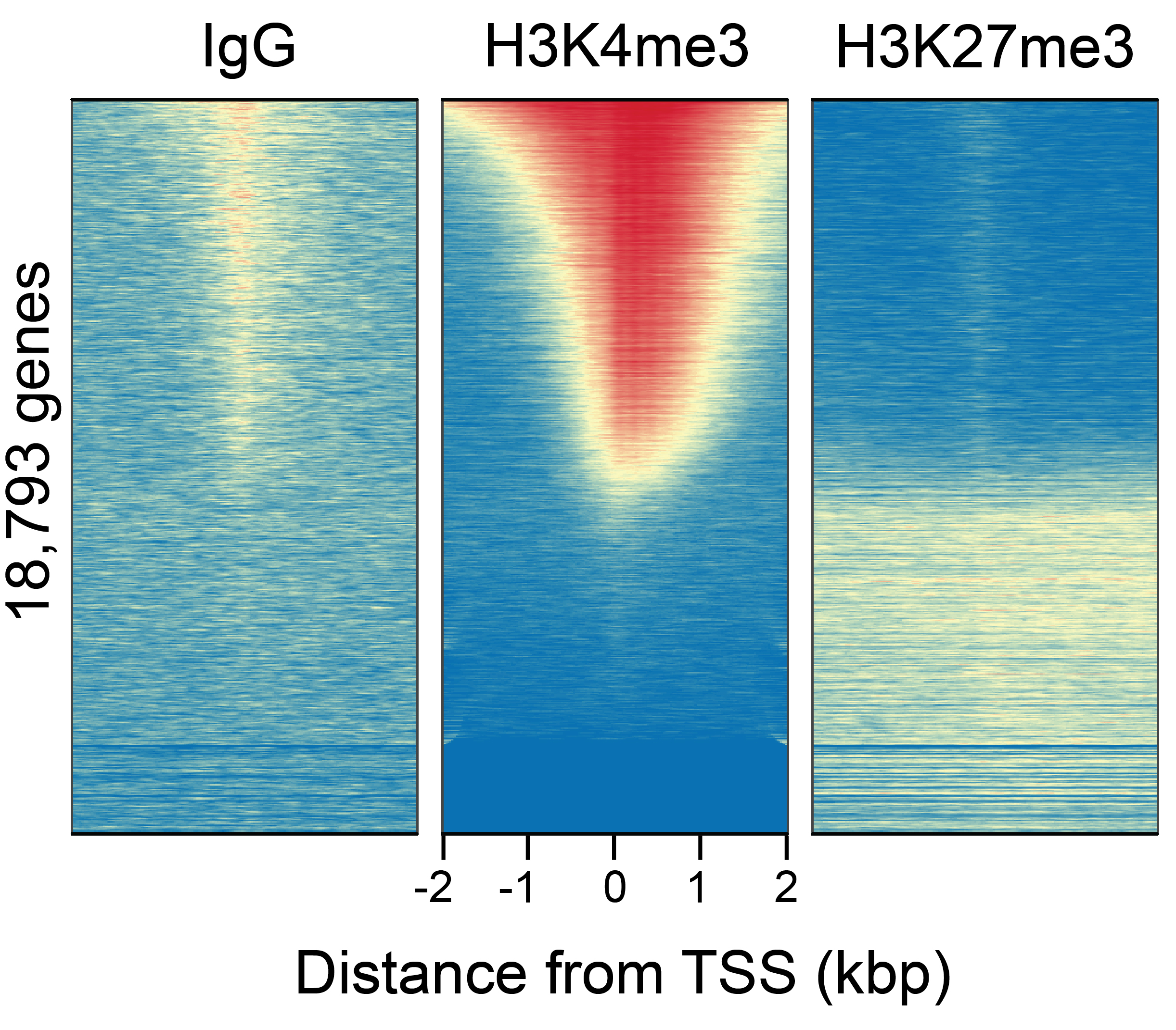
Figure 3: CUT&RUN genome-wide heatmaps
CUT&RUN was performed as described above. Heatmaps show
CUT&RUN signal aligned to annotated transcription start
sites (TSS, +/- 2kb). High and low signal are ranked by
intensity (top to bottom) and colored such that red
indicates high localized enrichment and blue denotes
background signal. Gene rows in each heatmap are aligned and
sorted from high to low signal relative to H3K4me3 (middle).
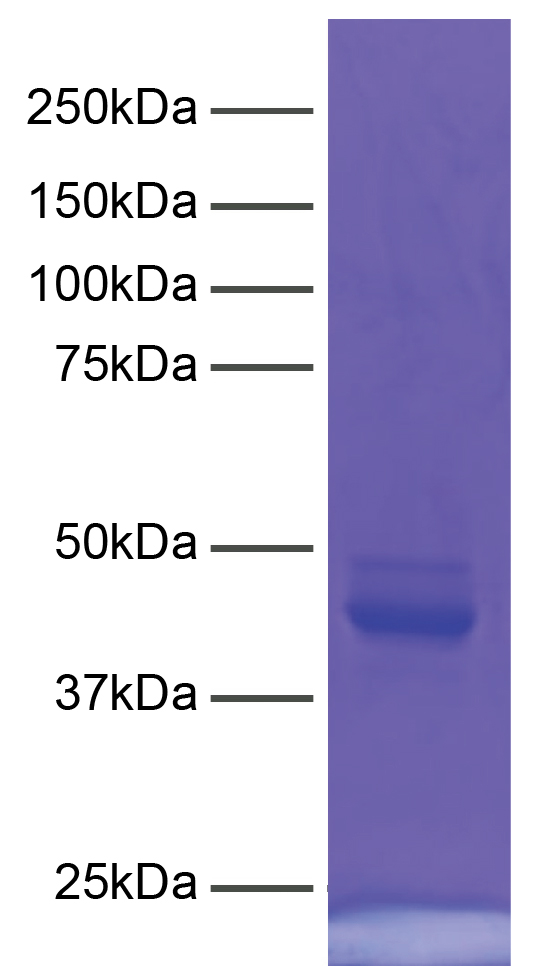
Figure 4: Protein gel data
CUTANA™ pAG-MNase (1 µg) was resolved via SDS-PAGE and
stained with Coomassie blue. The migration and molecular
weight of the protein standards are indicated.
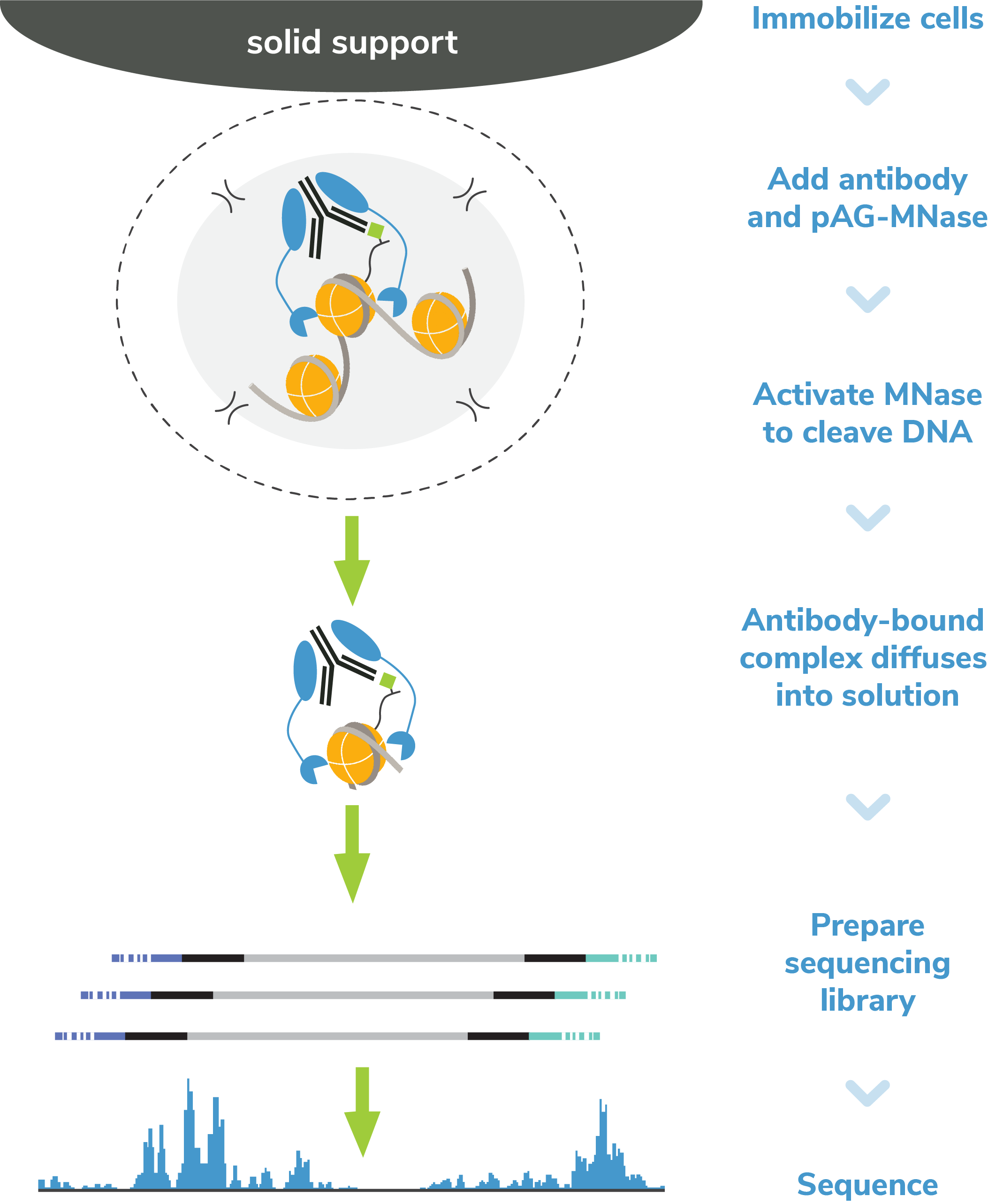
CUT&RUN methods
CUT&RUN was performed on 500k K562 cells with 0.5 µg of either IgG (EpiCypher 13-0042),
H3K4me3 (EpiCypher 13-0041), or H3K27me3 (ThermoFisher MA5-11198) antibodies
using CUTANA™ pAG-MNase (1:20 dilution) and the CUTANA™ ChIC/CUT&RUN Kit v3 (EpiCypher 14-1048).
Library preparation was performed with 5 ng of DNA (or the total amount recovered if less than 5 ng)
using the CUTANA™ CUT&RUN Library Prep Kit (EpiCypher 14-1001/14-1002). Both kit protocols were adapted for
high throughput Tecan liquid handling. Libraries were run on an Illumina NextSeq2000 with paired-end sequencing (2x50 bp).
Sample sequencing depth was 3.6 million reads (IgG), 4.3 million reads (H3K4me3), and 5.2 million reads (H3K27me3).
Data were aligned to the hg19 genome using Bowtie2. Data were filtered to remove duplicates, multi-aligned reads,
and ENCODE DAC Exclusion List regions.
Recommended Accessory Products
| Item | Cat. No. |
|---|---|
| CUTANA™ CUT&RUN Library Prep Kit | 14-1001 |
| CUTANA™ DNA Purification Kit | 14-0050 |
| CUTANA™ Concanavalin A Conjugated Paramagnetic Beads | 21-1401 |
| SNAP-CUTANA™ K-MetStat Panel | 19-1002 |
| CUT&RUN Antibodies | See the list |
| CUTANA™ E. coli Spike-in DNA | 18-1401 |
| Magnetic Separation Rack, 0.2 mL Tubes | 10-0008 |
| Magnetic Separation Rack, 1.5 mL Tubes | 10-0012 |
| CUTANA™ CUT&RUN 8-strip 0.2 mL Tubes | 10-0009 |
| CUTANA™ Nuclei Extraction Buffer | 21-1026 |
Technical Information
Application Notes
Add 2.5 µL of the supplied enzyme to a 50 µL CUT&RUN reaction (20X dilution). For detailed applications and uses of this product, please see our CUT&RUN protocol.