Mononucleosomes (H3.3G34W), Recombinant Human Biotinylated
In stock
Recombinant human histone H3.3 (H3F3A, H3.3A, H3F3, accession P84243) containing a methionine at position 4, expressed in E. coli. Histone H3 is one of the four proteins that are present in the nucleosome, the basic repeating unit subunit of chromatin, consisting of 147 base pairs of DNA wrapped around an octamer of core histone proteins (H2A, H2B, H3 and H4). H3.3 is a histone variant, a non-allelelic replacement histone found in regions of high chromatin turnover outside of S-phase (e.g. at actively transcribed genes). The substitution of W for G at position 34 is associated with pediatric diffuse intrinsic pontine gliomas (DIPGs) with and diminished levels of H3K36 methylation. The 601 sequence, identified by Lowary and Widom, is a 147-base pair sequence that has high affinity for histone octamers and is useful for nucleosome assembly and contains a 5′ biotin-TEG group.
Western Blot Data
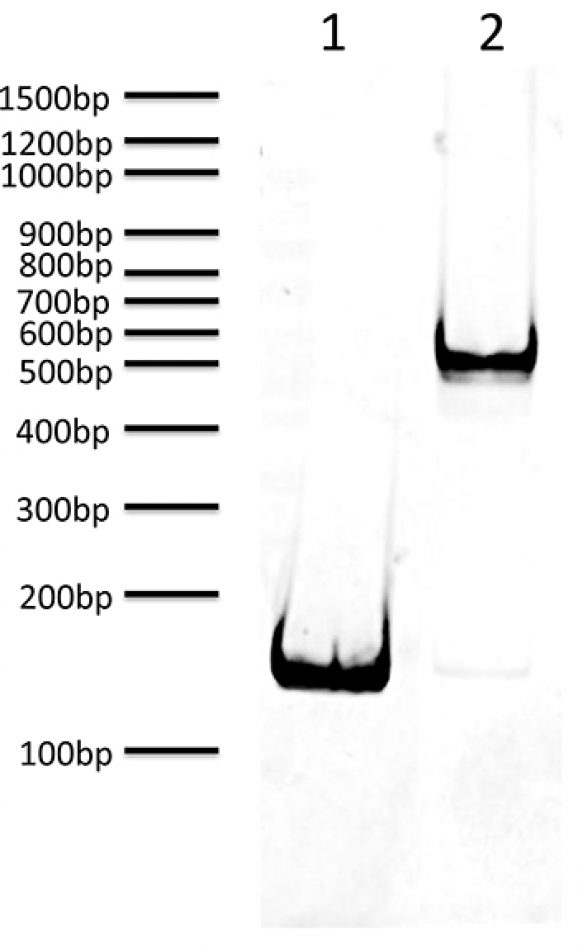
Mononucleosomes (H3.4G34W), Recombinant Human. Top Panel: Wildtype H3.3 (Lane 1) and H3.3G34W containing nucleosomes (Lane 2) were probed with an anti-H3.3G34W antibody. Only the H3.3G34W sample produced a detectable signal. Bottom Panel: Detail from Coomassie stained gel of Western blot.
Protein Gel Data:
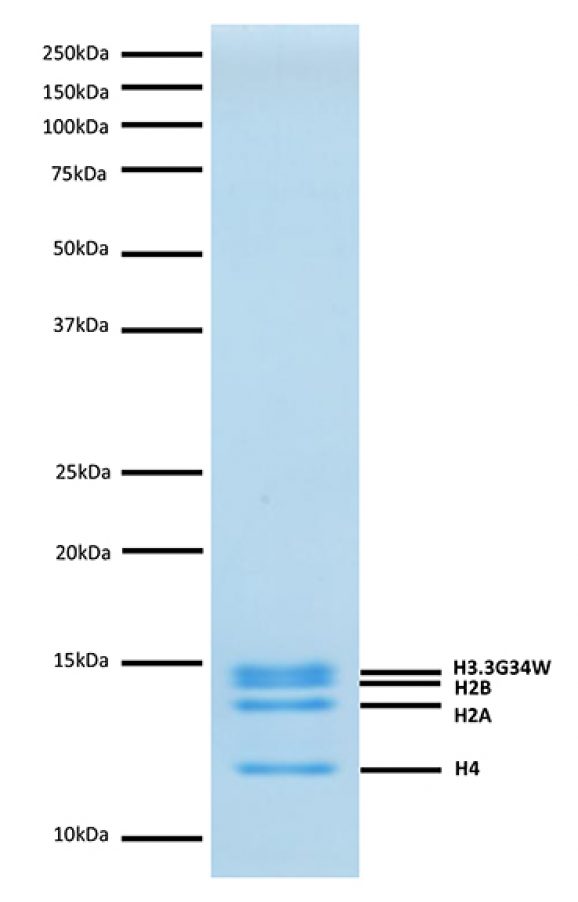
Coomassie stained PAGE gel of proteins in Mononucleosomes (H3.3 G34W), Recombinant Human (1 µg) to demonstrate the purity of the histones in the preparation. Sizes of molecular weight markers and positions of the core histones (H2A, H2B, H3.3G34W and H4) are indicated.
Mononucleosomes (H3.3G34W), Recombinant Human (27.3 µg protein weight, 50 µg DNA+protein) in 10 mM Tris HCl, pH 7.5, 25 mM NaCl, 1 mM EDTA, 2 mM DTT, 20% glycerol. MW = 200,621.
Application Notes
Mononucleosomes (H3.3G34W), Recombinant Human are highly purified and suitable for use as substrates in enzyme screening assays, structural studies, or effector protein binding experiments. Mononucleosomes (H3.3G34W), Recombinant Human from EpiCypher do not contain free DNA which could alter assayed activities.