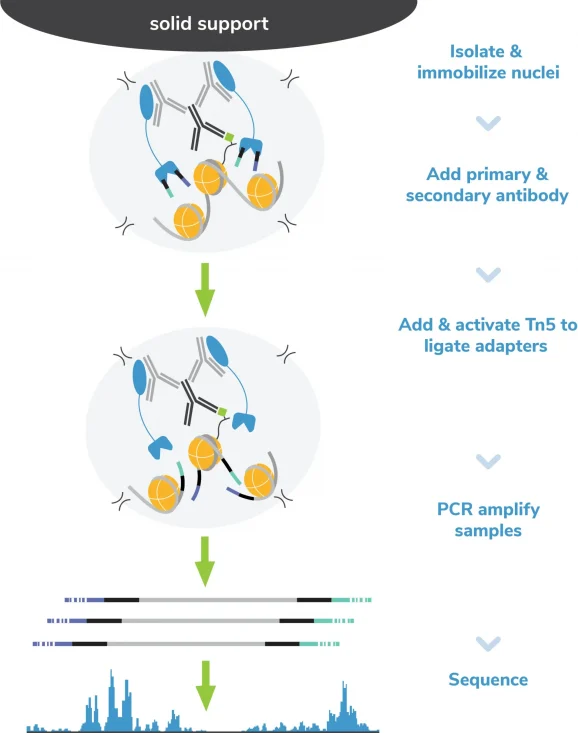
H3K27ac Antibody, SNAP-Certified™ for CUT&RUN and CUT&Tag
In stock
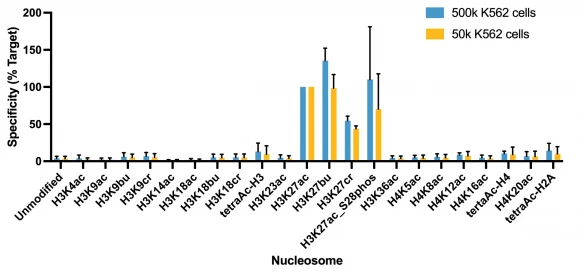
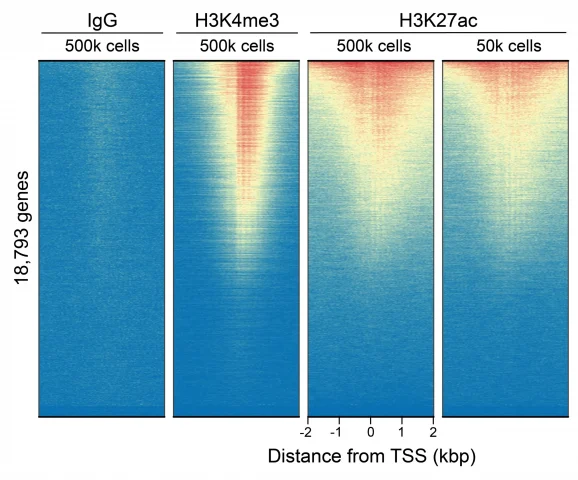
This H3K27ac (histone H3 acetylated at lysine 27) antibody meets EpiCypher’s lot-specific SNAP-Certified™ criteria for specificity and efficient target enrichment in both CUT&RUN and CUT&Tag applications. This requires <20% cross-reactivity to related histone PTMs determined using the SNAP-CUTANA™ K-AcylStat Panel of spike-in controls (EpiCypher RD193002, Figures 1 and 5). High target efficiency is confirmed by consistent genomic enrichment at varying cell inputs: 500k and 50k cells in CUT&RUN (Figures 2-3); 100k and 10k cells in CUT&Tag (Figures 6-7). High efficiency antibodies display similar peak structures (Figures 3 and 7) and highly conserved genome-wide signal (Figures 2 and 6) even at reduced cell numbers. H3K27ac is associated with gene activation and is enriched at active enhancers and promoters [Pei et al., 2020].
Validation Data
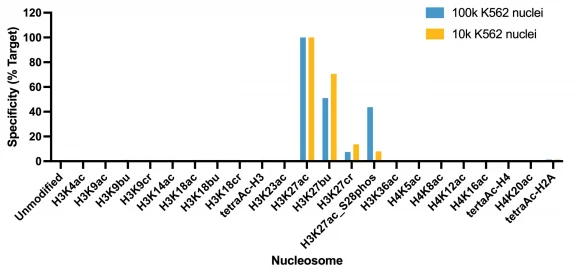
Figure 1: Average SNAP specificity analysis from two CUT&RUN experiments
Figure 2: CUT&RUN genome-wide enrichment
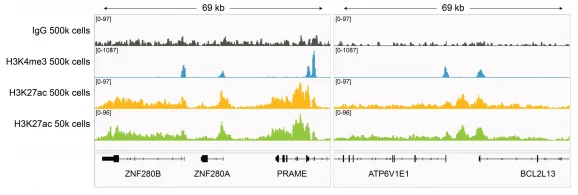
Figure 3: H3K27ac CUT&RUN representative browser tracks
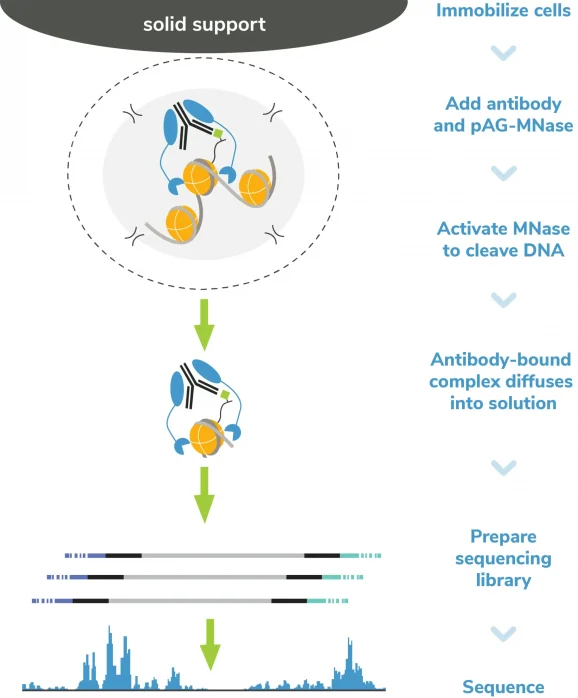
Figure 4: CUT&RUN methods
CUT&RUN was performed on 500k and 50k K562 cells with the SNAP-CUTANA™ K-MetStat Panel (EpiCypher 19-1002) or SNAP-CUTANA™ K-AcylStat Panel (EpiCypher RD193002) spiked-in prior to the addition of 0.5 µg of either IgG negative control (EpiCypher 13-0042), H3K4me3 positive control (EpiCypher 13-0041), or H3K27ac antibodies. The experiment was performed using the CUTANA™ ChIC/CUT&RUN Kit v3 (EpiCypher 14-1048). Library preparation was performed with 5 ng of CUT&RUN enriched DNA (or the total amount recovered if less than 5 ng) using the CUTANA™ CUT&RUN Library Prep Kit (EpiCypher 14-1001/14-1002). Both kit protocols were adapted for high throughput Tecan liquid handling. Libraries were run on an Illumina NextSeq2000 with paired-end sequencing (2×50 bp). Sample sequencing depth was 3.8 million reads (IgG 500k cell input), 5.0 million reads (IgG 50k cell input), 2.5 million reads (H3K4me3 500k cell input), 9.1 million reads (H3K4me3 50k cell input), 12.6 million reads (H3K27ac 500k cell input), and 11.0 million reads (H3K27ac 50k cell input). Data were aligned to the hg19 genome using Bowtie2. Data were filtered to remove duplicates, multi-aligned reads, and ENCODE DAC Exclusion List regions.
Figure 5: SNAP specificity analysis in CUT&Tag
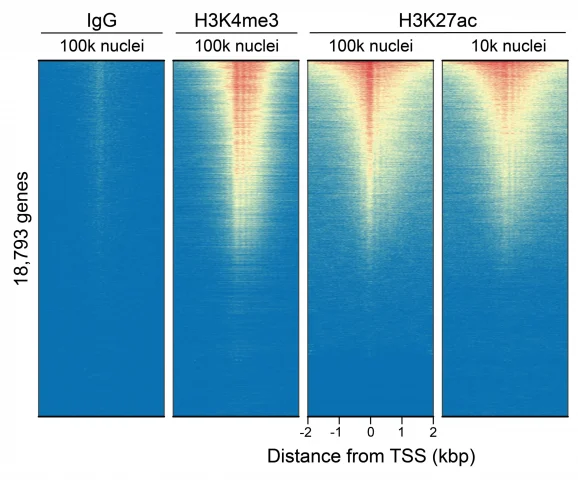
Figure 6: CUT&Tag genome-wide enrichment
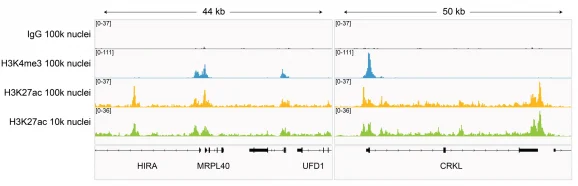
Figure 7: H3K27ac CUT&Tag representative browser tracks
Figure 8: CUT&Tag methods
CUT&Tag was performed on 100k and 10k K562 nuclei with the SNAP-CUTANA™ K-MetStat Panel (EpiCypher 19-1002) or SNAP-CUTANA™ K-AcylStat Panel (EpiCypher RD193002) spiked-in prior to the addition of 0.5 µg of either IgG negative control (EpiCypher 13-0042), H3K4me3 positive control (EpiCypher 13-0041), or H3K27ac antibodies. The experiment was performed using the CUTANA™ CUT&Tag Kit v1 (EpiCypher 14-1102/14-1103). Libraries were run on an Illumina NextSeq2000 with paired-end sequencing (2×50 bp). Sample sequencing depth was 1.3 million reads (IgG 100k nuclei input), 2.0 million reads (IgG 10k nuclei input), 3.5 million reads (H3K4me3 100k nuclei input), 8.0 million reads (H3K4me3 10k nuclei input), 8.1 million reads (H3K27ac 100k nuclei input) and 8.5 million reads (H3K27ac 10k nuclei input). Data were aligned to the hg19 genome using Bowtie2. Data were filtered to remove duplicates, multi-aligned reads, and ENCODE DAC Exclusion List regions.
- Type: Monoclonal [2114-3E4]
- Host: Rabbit
- Applications: CUT&RUN, CUT&Tag
- Reactivity: Human, Wide Range (Predicted)
- Format: Protein A affinity-purified
- Target Size: 15 kDa
Immunogen
A synthetic peptide corresponding to histone H3 acetylated at lysine 27
Formulation
Protein A affinity-purified recombinant monoclonal antibody in borate buffered saline pH 8.0, 0.09% sodium azide
- CUT&RUN: 0.5 µg per reaction
- CUT&Tag: 0.5 µg per reaction
- Uniprot ID: H3.1 – P68431
- Alternate Names: H3, H3/a, H3/b, H3/d